Targeting Novel Neural Circuits to Treat Major Conditions with High Unmet Need
Pipeline
Neurological Disorders
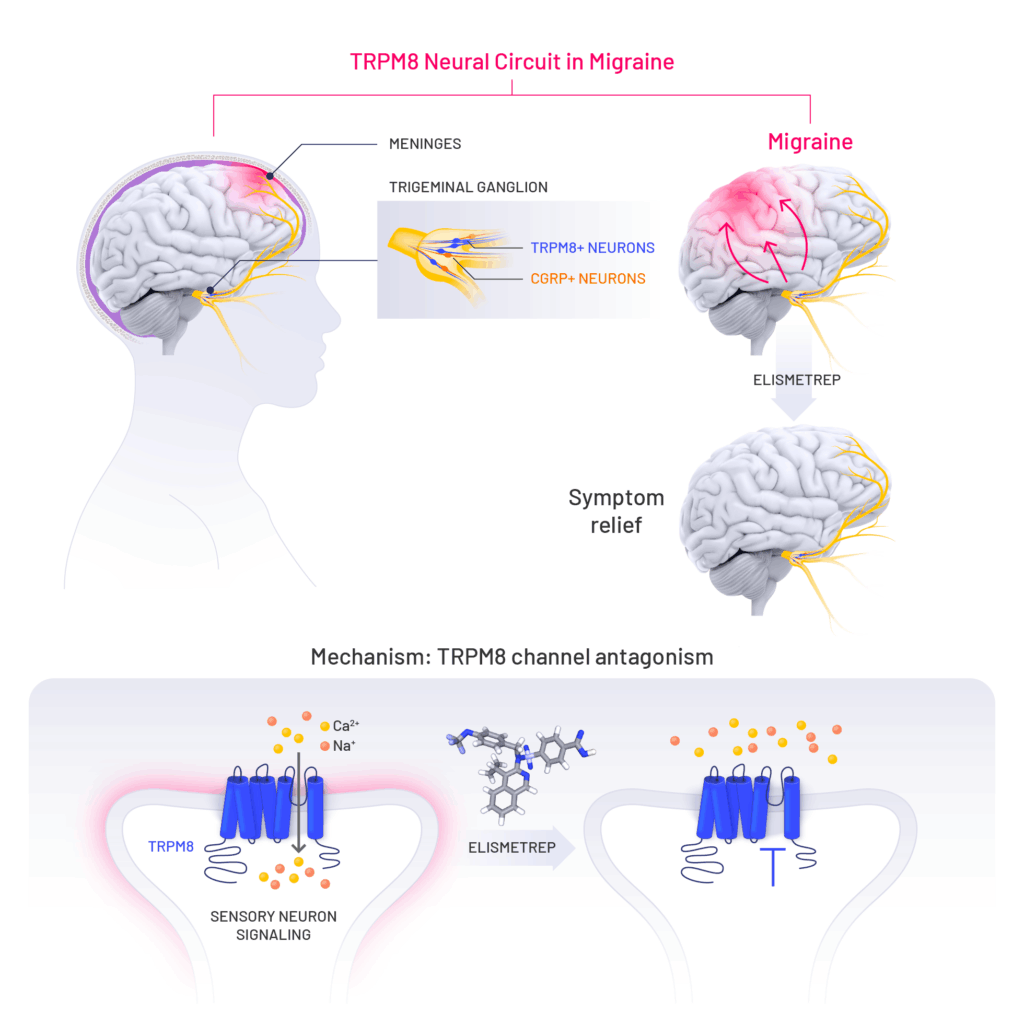
Elismetrep (K-304) is an oral first-in-class antagonist of TRPM8, a novel mechanism for treating migraine.
Metabolism
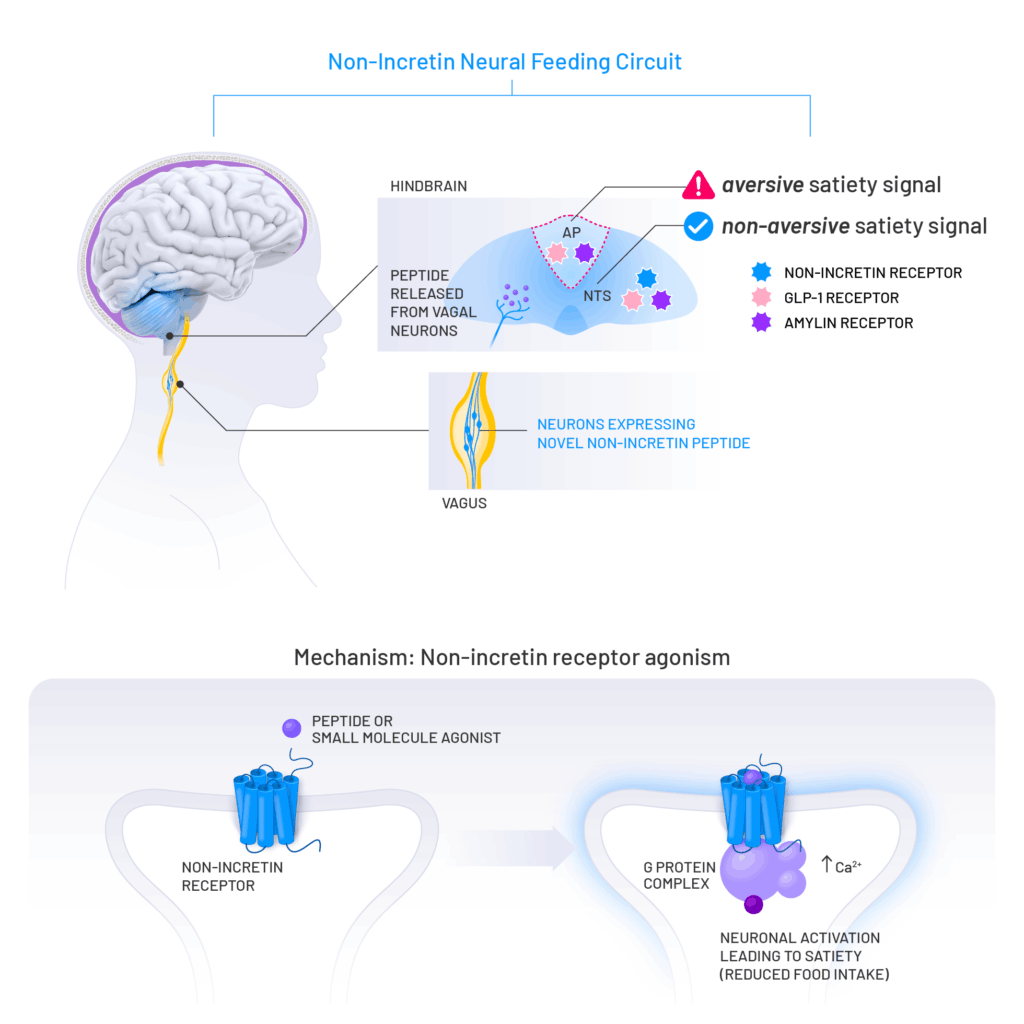
Kallyope is advancing a first-in-class non-incretin approach to metabolic disorders identified by the Klarity Platform. This unique feeding circuit is distinct from GLP-1 and amylin receptor biology.
Programs
Elismetrep (K-304): Bringing Innovation to a Global Market Defined by Low Patient Satisfaction
Our first-in-class migraine candidate elismetrep (K-304) targeting TRPM8 represents an entirely new approach for the treatment of migraine. We will initiate pivotal studies with elismetrep in 2026.
Debilitating migraines that impede the ability to participate in daily life affect tens of millions and create an addressable market for the acute treatment of migraine that is expected to exceed $16 billion by 2033. Despite its size, the migraine market is in urgent need of mechanistic innovation. Migraine drugs fall into several classes with each only effective in a small proportion of patients – often inconsistently. The most recent class focuses on antagonizing the effects of the neuropeptide calcitonin gene-related peptide (CGRP). Patients often switch between available classes of medication to seek relief.
Elismetrep targets TRPM8, representing an entirely new approach for the treatment of migraine. TRPM8 is expressed in neurons distinct from those expressing CGRP, suggesting it could be used in a complementary way. TRPM8 biology is also entirely distinct from other members of the TRPM family of proteins, further differentiating Kallyope’s approach. If approved, TRPM8 antagonists would represent a major expansion of therapeutic options for migraine patients.
Our nearly 450-patient Phase 2b study strongly supports elismetrep’s clinical benefit to migraine sufferers.
K-554: A Differentiated Approach to Obesity via a Novel Neural Feeding Circuit
Incretin-based therapies like GLP-1s have transformed the treatment of type 2 diabetes and obesity. Despite their success, significant unmet needs remain to be addressed by more tolerable, efficacious and convenient next-generation therapeutics.
K-554 targets a novel, non-incretin pathway and is uniquely positioned to address many of the limitations associated with first generation therapies, including tolerability. Identified utilizing the Klarity™ Platform, this unique feeding circuit is distinct from GLP-1 and amylin receptor biology, thus K-554 is anticipated to be efficacious in both monotherapy and in combination with existing agents. The molecule enters clinical development in mid-2026 and its target profile, including good tolerability, is supported by strong human genetics and compelling preclinical pharmacology.
We also see a significant unmet need for convenient and affordable oral agents for the treatment of obesity and are currently developing small molecule agonists of this target. A complementary small molecule amylin program is progressing in parallel.